
SPI Microanalysis Standards
Further information about Faraday Cups
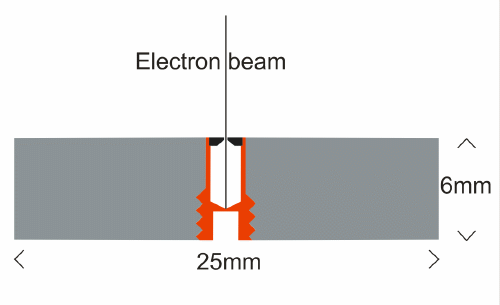
A Faraday Cup allows a beam of charged particles (electrons, ions) to be measured
accurately. It collects all the particles which enter it, and leads them to an
ammeter, or more correctly a micro-ammeter. Most electron beam instruments have
a Faraday Cup by which one can measure the electron beam current in the column,
but this is not the same as the current which strikes the sample. When
electrons hit the sample, some are back-scattered, and some are absorbed. The
relative proportion of back- scattered and absorbed electrons depends on the
nature of the sample material. Light materials have low back-scatter
coefficients; heavy materials, like gold, back-scatter a large proportion of the
beam.
The true reading of the current reaching the sample can only be determined if the
back-scattered proportion is reduced to near zero, thus forcing all the electrons
to be absorbed by the sample. A Faraday Cup accomplishes this by trapping the
electrons inside a cavity from which they have little chance of escaping. In the
SPI Faraday Cup, which is included with every standard mount we produce, the
electron beam is directed through a 200 micrometer aperture in a piece of
platinum. Below this aperture is a relatively large stainless steel cavity in which
electrons may bounce around, ultimately coming to rest in the walls, whence
they are conducted away through the sample to the sample current meter. The
cup has a screw thread which allows it to be inserted and removed easily using
a hex Allen key.
Why should one want to know the current incident on the sample? Really this is a
quantity which should be reported when analyses are made, not only for
comparative purposes from day to day, but also to ensure that the instrument is
functioning correctly. If you know the true current, measured by Faraday Cup,
and you also know the absorbed current in a particular material, let us say X,
then the difference is the back-scattered current, which is a function of the
atomic number, Z, of X. Hence Faraday Cup measurements allow you to determine
the mean Z of a sample independently of knowing its chemical composition.

 To Ask a Question or Make a Comment
To Ask a Question or Make a Comment
 To Place an Order or Request a Quote
To Place an Order or Request a Quote
Return to:
Friday October 03, 2008
© Copyright 1999 - 2008. By Structure Probe, Inc.
Contacting SPI Supplies and Structure Probe, Inc.
All rights reserved.
All trademarks and trade names are the property of their respective owners.
Privacy Policy
Worldwide Distributors, Representatives, and Agents


 To Ask a Question or Make a Comment
To Ask a Question or Make a Comment


 To Ask a Question or Make a Comment
To Ask a Question or Make a Comment To Place an Order or Request a Quote
To Place an Order or Request a Quote